Precision medicine continues to gain traction, and increasingly, clinicians are turning to comprehensive genomic profiling to match the right drug to a patient using information about that patient’s genetic mutations.
Yet despite the growing opportunities and potential for comprehensive genomic profiling in immuno-oncology, there remain challenges, many of which have to do with the limitations associated with accessing tumor tissue. Traditional tissue biopsies are expensive and approximately 30% of the time, they don’t yield enough tissue. [1] And most importantly, they are invasive procedures which carry complication risks for patients.
Liquid biopsy-based approaches overcome many of these challenges but since most healthcare institutions must currently perform these tests as send-outs, they relinquish control over turnaround time and miss the opportunity for collaboration between ordering physicians and pathologists who share the same roof.
To address the challenges inherent in some traditional tissue biopsies and to enable healthcare institutions to perform their own liquid biopsy tests, Illumina has recently launched the TruSight Oncology 500 ctDNA assay. We are proud to announce that we provide full support for this assay.
Overview of the Workflow
TruSight Oncology 500 ctDNA is a multiplex assay that analyzes cancer-related biomarkers from plasma simultaneously. The assay also assesses immuno-oncology and emerging biomarkers, such as TMB, MSI, NTRK, and ROS1. [2]
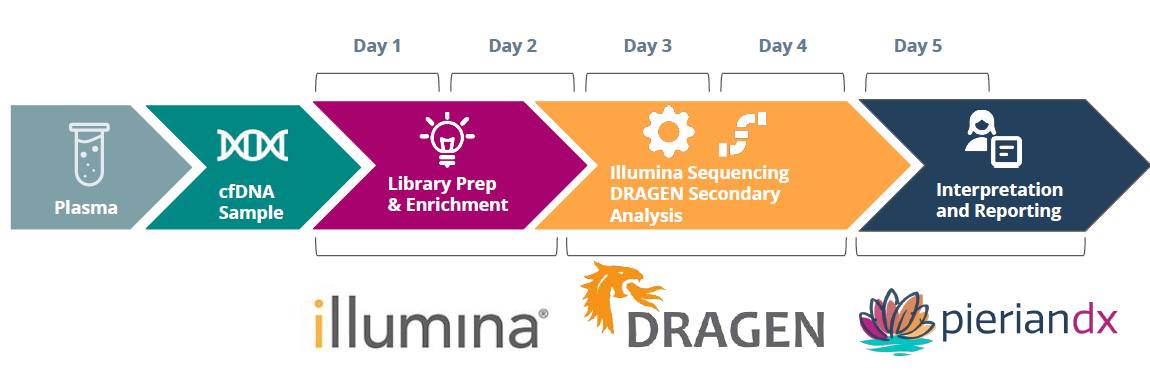
After cell-free DNA (cfDNA) is extracted from plasma, library prep and enrichment are performed using the Illumina TruSight Oncology 500 ctDNA kit. Samples are then sequenced on the NovaSeq 6000 system and secondary analysis is performed using the Dragen software on the Dragen v3 server. Users can then upload VCF files directly into PierianDx Clinical Genomics Workspace to create a draft report that can then be signed out by a medical professional. From start to finish, the process takes only five days.
For more information, contact us.
References
- Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC, Rolfo, Christian et al. Journal of Thoracic Oncology, Volume 13, Issue 9, 1248 - 1268
- TruSight™ Oncology 500 ctDNA. TruSight™ Oncology 500 ctDNA.

